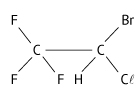
Considere a fórmula estrutural do anestésico geral halotano (massa molar aproximada 200 g/mol).

a) Escreva a fórmula molecular do halotano e calcule a porcentagem em massa de flúor nesse anestésico. Apresente os cálculos.
b) O halotano deve apresentar isomeria geométrica (cis-trans)? E isomeria óptica? Justifique suas respostas.
a)
Fórmula molecular
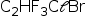
Massa molar = 197,5 g/mol
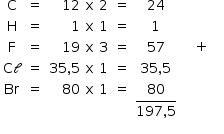
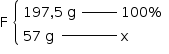
x = 28,86%
b) O halotano apresenta isomeria óptica, pois possui carbono quiral (assimétrico):
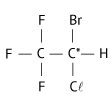
Não apresenta isomeria geométrica, pois compostos acíclicos devem conter dupla-ligação entre carbonos e ligantes diferentes nos carbonos da dupla-ligação.